Aluminium-26 (26 Al, Al-26) is a radioactive isotope of the chemical element aluminium, decaying by either positron emission or electron capture to stable magnesium-26.The half-life of 26 Al is 7.17 × 10 5 years. This is far too short for the isotope to survive as a primordial nuclide, but a small amount of it is produced by collisions of atoms with cosmic ray protons. Atomic number 16 is Sulfur: It’s in period 3, group 6/16, and considered a nonmetal. It has 6 valence electrons in it’s outer shell for the Rutherford Model, On the Quantum Mechanical Model it has two unpaired electrons. Definitions of atomic number 26. A heavy ductile magnetic metallic element; is silver-white in pure form but readily rusts; used in construction and tools and armament; plays a role in the transport of oxygen by the blood. Synonyms: Fe, iron.

| General | |
|---|---|
| Symbol | 26Al |
| Names | aluminium-26, Al-26 |
| Protons | 13 |
| Neutrons | 13 |
| Nuclide data | |
| Natural abundance | trace (cosmogenic) |
| Half-life | 7.17×105 years |
| Spin | 5+ |
| Decay modes | |
| Decay mode | Decay energy (MeV) |
| β+ | 4.00414 |
| ε | 4.00414 |
| Isotopes of aluminium Complete table of nuclides | |
Aluminium-26 (26Al, Al-26) is a radioactive isotope of the chemical elementaluminium, decaying by either positron emission or electron capture to stable magnesium-26. The half-life of 26Al is 7.17×105 years. This is far too short for the isotope to survive as a primordial nuclide, but a small amount of it is produced by collisions of atoms with cosmic rayprotons.[1]
Decay of aluminium-26 also produces gamma rays and x-rays.[2] The x-rays and Auger electrons are emitted by the excited atomic shell of the daughter 26Mg after the electron capture which typically leaves a hole in one of the lower sub-shells.

Because it is radioactive, it is typically stored behind at least 5 centimetres (2 in) of lead. Contact with 26Al may result in radiological contamination necessitating special tools for transfer, use, and storage.[3]
Dating[edit]

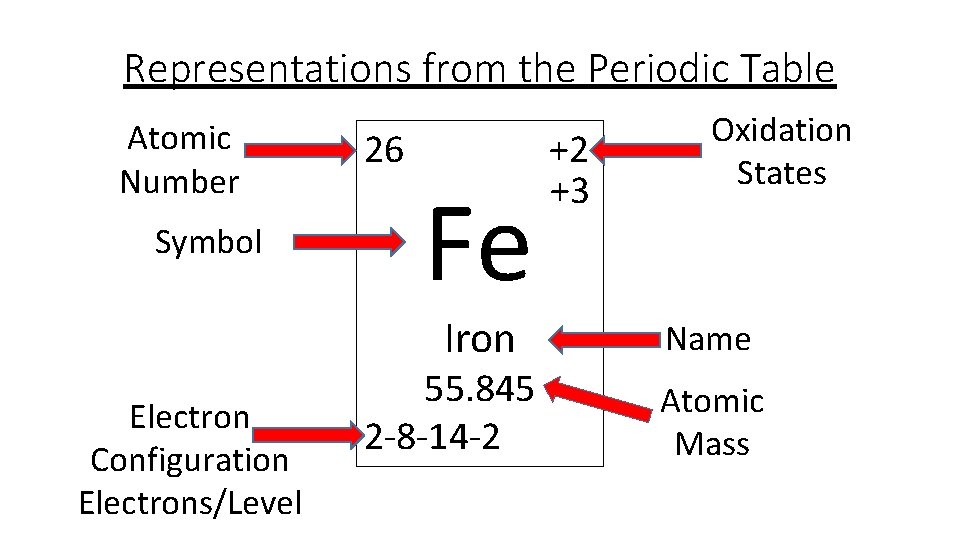
Element With 26 Atomic Number
Aluminium-26 can be used to calculate the terrestrial age of meteorites and comets. It is produced in significant quantities in extraterrestrial objects via spallation of silicon alongside beryllium-10, though after falling to Earth, 26Al production ceases and its abundance relative to other cosmogenic nuclides decreases. Absence of aluminium-26 sources on Earth is a consequence of Earth's atmosphere obstructing silicon on the surface and low troposphere from interaction with cosmic rays. Consequently, the amount of 26Al in the sample can be used to calculate the date the meteorite fell to Earth.[1]
Occurrence in the interstellar medium[edit]
The gamma emission at 1809 keV was the first observed gamma emission from the galactic center. The observation was made by the HEAO-3 satellite in 1984.[4][5]
The isotope is mainly produced in supernovas ejecting many radioactive nuclides in the interstellar medium. The isotope is believed to provide enough heat to small planetary bodies so as to differentiate their interiors, such as has been the case in the early history of the asteroids 1 Ceres and 4 Vesta.[6][7][8] This isotope also features in hypotheses regarding the equatorial bulge of Saturn's moon Iapetus.[9]
History[edit]
Before 1954, the half-life of aluminium-26 was measured to be 6.3 seconds.[10] After it was theorized that this could be the half-life of a metastable state (isomer) of aluminium-26, the ground state was produced by bombardment of magnesium-26 and magnesium-25 with deuterons in the cyclotron of the University of Pittsburgh.[11] The first half-life was determined to be in the range of 106 years.
The Fermi beta decay half-life of the aluminium-26 metastable state is of interest in the experimental testing of two components of the Standard Model, namely, the conserved-vector-current hypothesis and the required unitarity of the Cabibbo–Kobayashi–Maskawa matrix.[12] The decay is superallowed. The 2011 measurement of the half life of 26mAl is 6346.54 ± 0.46(statistical) ± 0.60(system) milliseconds.[13] In considering the known melting of small planetary bodies in the early Solar System, H. C. Urey noted that the naturally occurring long-lived radioactive nuclei (40K, 238U, 235U and 232Th) were insufficient heat sources. He proposed that the heat sources from short lived nuclei from newly formed stars might be the source and identified 26Al as the most likely choice.[14][15] This proposal was made well before the general problems of stellar nucleosynthesis of the nuclei were known or understood. This conjecture was based on the discovery of 26Al in a Mg target by Simanton, Rightmire, Long & Kohman.[11]
Their search was undertaken because hitherto there was no known radioactive isotope of Al that might be useful as a tracer. Theoretical considerations suggested that a state of 26Al should exist. The life time of 26Al was not then known; it was only estimated between 104 and 106 years. The search for 26Al took place over many years, long after the discovery of the extinct radionuclide129I (by Reynolds (1960, Physical Review Letters v 4, p 8)) which showed that contribution from stellar sources formed ~108 years before the Sun had contributed[how?] to the Solar System mix. The asteroidal materials that provide meteorite samples were long known to be from the early Solar System.[16]
The Allende meteorite, which fell in 1969, contained abundant calcium–aluminium-rich inclusions (CAIs). These are very refractory materials and were interpreted as being condensates from a hot solar nebula.[17][18] then discovered that the oxygen in these objects was enhanced in 16O by ~5% while the 17O/18O was the same as terrestrial. This clearly showed a large effect in an abundant element that might be nuclear, possibly from a stellar source. These objects were then found to contain strontium with very low 87Sr/86Sr indicating that they were a few million years older than previously analyzed meteoritic material and that this type of material would merit a search for 26Al.[19]26Al is only present today in the Solar System materials as the result of cosmic reactions on unshielded materials at an extremely[quantify] low level. Thus, any original 26Al in the early Solar System is now extinct.
To establish the presence of 26Al in very ancient materials requires demonstrating that samples must contain clear excesses of 26Mg /24Mg which correlates with the ratio of 27Al/24Mg. The stable 27Al is then a surrogate for extinct 26Al. The different 27Al/24Mg ratios are coupled to different chemical phases in a sample and are the result of normal chemical separation processes associated with the growth of the crystals in the CAIs. Clear evidence of the presence of 26Al at an abundance ratio of 5×10−5 was shown by Lee, et al.[20][21] The value (26Al/27Al ∼ 5×10−5) has now been generally established as the high value in early Solar System samples and has been generally used as a refined time scale chronometer for the early Solar System. Lower values imply a more recent time of formation. If this 26Al is the result of pre-solar stellar sources, then this implies a close connection in time between the formation of the Solar System and the production in some exploding star. Many materials which had been presumed to be very early (e.g. chondrules) appear to have formed a few million years later (Hutcheon & Hutchison)[citation needed]. Other extinct radioactive nuclei, which clearly had a stellar origin, were then being discovered.[22]
That 26Al was present in the interstellar medium as a major gamma ray source was not explored until the development of the high-energy astronomical observatory program. The HEAO-3 spacecraft with cooled Ge detectors allowed the clear detection of 1.808 Mev gamma lines from the central part of the galaxy from a distributed of 26Al source.[4] This represents a quasi steady state inventory corresponding to two solar masses of 26Al was distributed[clarification needed]. This discovery was greatly expanded on by observations from the Compton Gamma Ray Observatory using the COMPTEL telescope in the galaxy.[23] Subsequently, the 60Fe lines (1.173 & 1.333 Mev) were also detected showing the relative rates of decays from 60Fe to 26Al to be 60Fe/26AL~0.11.[24]
In pursuit of the carriers of 22Ne in the sludge produced by chemical destruction of some meteorites, carrier grains in micron size, acid-resistant ultra-refractory materials (e.g. C, SiC) were found by E. Anders & the Chicago group. The carrier grains were clearly shown to be circumstellar condensates from earlier stars and often contained very large enhancements in 26Mg/24Mg from the decay of 26Al with 26Al/27Al sometimes approaching 0.2 [25][26] These studies on micron scale grains were possible as a result of the development of surface ion mass spectrometry at high mass resolution with a focused beam developed by G. Slodzian & R.Castaing with the CAMECA Co.
The production of 26Al by cosmic ray interactions in unshielded materials is used as a monitor of the time of exposure to cosmic rays. The amounts are far below the initial inventory that is found in very early solar system debris.
See also[edit]
References[edit]
- ^ abOverholt, A.C.; Melott, A.L. (2013). 'Cosmogenic nuclide enhancement via deposition from long-period comets as a test of the Younger Dryas impact hypothesis'. Earth and Planetary Letters. 377–378: 55–61. arXiv:1307.6557. Bibcode:2013E&PSL.377...55O. doi:10.1016/j.epsl.2013.07.029. S2CID119291750.
- ^'Nuclide Safety Data Sheet Aluminum-26'(PDF). www.nchps.org.
- ^'Nuclide Safety Data Sheet Aluminum-26'(PDF). National Health& Physics Society. Retrieved 2009-04-13.CS1 maint: discouraged parameter (link)
- ^ abMahoney, W. A.; Ling, J. C.; Wheaton, W. A.; Jacobson, A. S. (1984). 'HEAO 3 discovery of Al-26 in the interstellar medium'. The Astrophysical Journal. 286: 578. Bibcode:1984ApJ...286..578M. doi:10.1086/162632.
- ^Kohman, T. P. (1997). 'Aluminum-26: A nuclide for all seasons'. Journal of Radioanalytical and Nuclear Chemistry. 219 (2): 165–176. doi:10.1007/BF02038496. S2CID96683475.
- ^Moskovitz, Nicholas; Gaidos, Eric (2011). 'Differentiation of planetesimals and the thermal consequences of melt migration'. Meteoritics & Planetary Science. 46 (6): 903–918. arXiv:1101.4165. Bibcode:2011M&PS...46..903M. doi:10.1111/j.1945-5100.2011.01201.x. S2CID45803132.
- ^Zolotov, M. Yu. (2009). 'On the Composition and Differentiation of Ceres'. Icarus. 204 (1): 183–193. Bibcode:2009Icar..204..183Z. doi:10.1016/j.icarus.2009.06.011.
- ^Zuber, Maria T.; McSween, Harry Y.; Binzel, Richard P.; Elkins-Tanton, Linda T.; Konopliv, Alexander S.; Pieters, Carle M.; Smith, David E. (2011). 'Origin, Internal Structure and Evolution of 4 Vesta'. Space Science Reviews. 163 (1–4): 77–93. Bibcode:2011SSRv..163...77Z. doi:10.1007/s11214-011-9806-8. S2CID7658841.
- ^Kerr, Richard A. (2006-01-06). 'How Saturn's Icy Moons Get a (Geologic) Life'. Science. 311 (5757): 29. doi:10.1126/science.311.5757.29. PMID16400121. S2CID28074320.
- ^Hollander, J. M.; Perlman, I.; Seaborg, G. T. (1953). 'Table of Isotopes'. Reviews of Modern Physics. 25 (2): 469–651. Bibcode:1953RvMP...25..469H. doi:10.1103/RevModPhys.25.469.CS1 maint: discouraged parameter (link)
- ^ abSimanton, James R.; Rightmire, Robert A.; Long, Alton L.; Kohman, Truman P. (1954). 'Long-Lived Radioactive Aluminum 26'. Physical Review. 96 (6): 1711–1712. Bibcode:1954PhRv...96.1711S. doi:10.1103/PhysRev.96.1711.
- ^Scott, Rebecca J; o'Keefe, Graeme J; Thompson, Maxwell N; Rassool, Roger P (2011). 'Precise measurement of the half-life of the Fermi β-decay of 26Al(m)'. Physical Review C. 84 (2): 024611. Bibcode:2011PhRvC..84b4611S. doi:10.1103/PhysRevC.84.024611.
- ^Finlay, P; Ettenauer, S; Ball, G. C; Leslie, J. R; Svensson, C. E; Andreoiu, C; Austin, R. A. E; Bandyopadhyay, D; Cross, D. S; Demand, G; Djongolov, M; Garrett, P. E; Green, K. L; Grinyer, G. F; Hackman, G; Leach, K. G; Pearson, C. J; Phillips, A. A; Sumithrarachchi, C. S; Triambak, S; Williams, S. J (2011). 'High-Precision Half-Life Measurement for the Superallowed β+ Emitter 26Al(m)'. Physical Review Letters. 106 (3): 032501. doi:10.1103/PhysRevLett.106.032501. PMID21405268.
- ^Urey, H.C. (1955). 'The Cosmic Abundances of Potassium, Uranium, and Thorium and the Heat Balances of the Earth, the Moon, and Mars'. PNAS. 41 (3): 127–144. Bibcode:1955PNAS...41..127U. doi:10.1073/pnas.41.3.127. PMC528039. PMID16589631.
- ^Urey, H.C. (1956). 'The Cosmic Abundances of Potassium, Uranium, and Thorium and the Heat Balances of the Earth, the Moon, and Mars'. PNAS. 42 (12): 889–891. Bibcode:1956PNAS...42..889U. doi:10.1073/pnas.42.12.889. PMC528364. PMID16589968.
- ^Black, D.C.; Pepin, R.O. (11 July 1969). 'Trapped neon in meteorites — II'. Earth and Planetary Science Letters. 6 (5): 395. Bibcode:1969E&PSL...6..395B. doi:10.1016/0012-821X(69)90190-3.
- ^Grossman, L. (June 1972). 'Condensation in the primitive solar nebula'. Geochimica et Cosmochimica Acta. 36 (5): 597. Bibcode:1972GeCoA..36..597G. doi:10.1016/0016-7037(72)90078-6.
- ^Clayton, Robert N.; Grossman, L.; Mayeda, Toshiko K. (2 November 1973). 'A component of primitive nuclear composition in carbonaceous meteorites'. Science. 182 (4111): 485–8. Bibcode:1973Sci...182..485C. doi:10.1126/science.182.4111.485. PMID17832468. S2CID22386977.CS1 maint: discouraged parameter (link)
- ^Gray (1973). 'The identification of early condensates from the solar nebula'. Icarus. 20 (2): 213. Bibcode:1973Icar...20..213G. doi:10.1016/0019-1035(73)90052-3.
- ^Lee, Typhoon; Papanastassiou, D. A; Wasserburg, G. J (1976). 'Demonstration of 26 Mg excess in Allende and evidence for 26 Al'. Geophysical Research Letters. 3 (1): 41. Bibcode:1976GeoRL...3...41L. doi:10.1029/GL003i001p00041.
- ^Lee, T.; Papanastassiou, D. A.; Wasserburg, G. J. (1977). 'Aluminum-26 in the early solar system - Fossil or fuel'. Astrophysical Journal Letters. 211: 107. Bibcode:1977ApJ...211L.107L. doi:10.1086/182351. ISSN2041-8205.
- ^Kelly; Wasserburg (December 1978). 'Evidence for the existence of 107Pd in the early solar system'. Geophysical Research Letters. 5 (12): 1079. Bibcode:1978GeoRL...5.1079K. doi:10.1029/GL005i012p01079. (t1/2=6.5x10^6 yr)
- ^Diehl, R.; Dupraz, C.; Bennett, K.; et al. (1995). 'COMPTEL observations of Galactic 26Al emission'. Astronomy & Astrophysics. 298: 445. Bibcode:1995A&A...298..445D.
- ^Harris, M. J.; Knödlseder, J.; Jean, P.; Cisana, E.; Diehl, R.; Lichti, G. G.; Roques, J.-P.; Schanne, S.; Weidenspointner, G. (29 March 2005). 'Detection of γ-ray lines from interstellar 60Fe by the high resolution spectrometer SPI'. Astronomy & Astrophysics. 433 (3): L49. arXiv:astro-ph/0502219. Bibcode:2005A&A...433L..49H. doi:10.1051/0004-6361:200500093. S2CID5358047.
- ^Anders, E.; Zinner, E. (September 1993). 'Interstellar grains in primitive meteorites: Diamond, silicon carbide, and graphite'. Meteoritics. 28 (4): 490–514. Bibcode:1993Metic..28..490A. doi:10.1111/j.1945-5100.1993.tb00274.x.
- ^Zinner, E. (2014). 'Presolar grains'. In H. D. Holland; K. K. Turekian; A. M. Davis (eds.). Treatise on Geochemistry. Treatise on Geochemistry, Second Edition. 1. pp. 181–213. doi:10.1016/B978-0-08-095975-7.00101-7. ISBN9780080959757.
Learning Objectives
- Define and differentiate between the atomic number and the mass number of an element.
- Explain how isotopes differ from one another.
Now that we know how atoms are generally constructed, what do atoms of any particular element look like? How many protons, neutrons, and electrons are in a specific kind of atom? First, if an atom is electrically neutral overall, then the number of protons equals the number of electrons. Because these particles have the same but opposite charges, equal numbers cancel out, producing a neutral atom.
Atomic Number
In the 1910s, experiments with x-rays led to this useful conclusion: the magnitude of the positive charge in the nucleus of every atom of a particular element is the same. In other words, all atoms of the same element have the same number of protons. Furthermore, different elements have a different number of protons in their nuclei, so the number of protons in the nucleus of an atom is characteristic of a particular element. This discovery was so important to our understanding of atoms that the number of protons in the nucleus of an atom is called the atomic number (Z).
For example, hydrogen has the atomic number 1; all hydrogen atoms have 1 proton in their nuclei. Helium has the atomic number 2; all helium atoms have 2 protons in their nuclei. There is no such thing as a hydrogen atom with 2 protons in its nucleus; a nucleus with 2 protons would be a helium atom. The atomic number defines an element. Table (PageIndex{1}) lists some common elements and their atomic numbers. Based on its atomic number, you can determine the number of protons in the nucleus of an atom. The largest atoms have over 100 protons in their nuclei.
| Element | Atomic Number | Element | Atomic Nmbers |
|---|---|---|---|
| aluminum (Al) | 13 | magnesium (Mg) | 12 |
| americium (Am) | 95 | manganese (Mn) | 25 |
| argon (Ar) | 18 | mercury (Hg) | 80 |
| barium (Ba) | 56 | neon (Ne) | 10 |
| beryllium (Be) | 4 | nickel (Ni) | 28 |
| bromine (Br) | 35 | nitrogen (N) | 7 |
| calcium (Ca) | 20 | oxygen (O) | 8 |
| carbon (C) | 6 | phosphorus (P) | 15 |
| chlorine (Cl) | 17 | platinum (Pt) | 78 |
| chromium (Cr) | 24 | potassium (K) | 19 |
| cesium (Cs) | 55 | radon (Rn) | 86 |
| fluorine (F) | 9 | silver (Ag) | 47 |
| gallium (Ga) | 31 | sodium (Na) | 11 |
| gold (Au) | 79 | strontium (Sr) | 38 |
| helium (He) | 2 | sulfur (S) | 16 |
| hydrogen (H) | 1 | titanium (Ti) | 22 |
| iron (Fe) | 26 | tungsten (W) | 74 |
| iodine (I) | 53 | uranium (U) | 92 |
| lead (Pb) | 82 | zinc (Zn) | 30 |
| lithium (Li) | 3 | zirconium (Zr) | 40 |
Example (PageIndex{1})
What is the number of protons in the nucleus of each element?
- aluminum
- iron
- carbon
According to Table 2.4.1, aluminum has an atomic number of 13. Therefore, every aluminum atom has 13 protons in its nucleus.
Iron has an atomic number of 26. Therefore, every iron atom has 26 protons in its nucleus.
Carbon has an atomic number of 6. Therefore, every carbon atom has 6 protons in its nucleus.
Exercise (PageIndex{1})
What is the number of protons in the nucleus of each element? Use Table 2.4.1.
- sodium
- oxygen
- chlorine

Sodium has 11 protons in its nucleus.
Oxygen has 8 protons in its nucleus.
Chlorine has 17 protons in its nucleus
How many electrons are in an atom? Previously we said that for an electrically neutral atom, the number of electrons equals the number of protons, so the total opposite charges cancel. Thus, the atomic number of an element also gives the number of electrons in an atom of that element. (Later we will find that some elements may gain or lose electrons from their atoms, so those atoms will no longer be electrically neutral. Thus we will need a way to differentiate the number of electrons for those elements.)
Example (PageIndex{2})
How many electrons are present in the atoms of each element?
- sulfur
- tungsten
- argon
The atomic number of sulfur is 16. Therefore, in a neutral atom of sulfur, there are 16 electrons.
The atomic number of tungsten is 74. Therefore, in a neutral atom of tungsten, there are 74 electrons.
The atomic number of argon is 18. Therefore, in a neutral atom of argon, there are 18 electrons.
Exercise (PageIndex{2})
How many electrons are present in the atoms of each element?
- magnesium
- potassium
- iodine
Mg has 12 electrons.
K has 19 electrons.
I has 53 electrons.
Isotopes
How many neutrons are in atoms of a particular element? At first it was thought that the number of neutrons in a nucleus was also characteristic of an element. However, it was found that atoms of the same element can have different numbers of neutrons. Atoms of the same element (i.e., same atomic number, Z) that have different numbers of neutrons are called isotopes. For example, 99% of the carbon atoms on Earth have 6 neutrons and 6 protons in their nuclei; about 1% of the carbon atoms have 7 neutrons in their nuclei. Naturally occurring carbon on Earth, therefore, is actually a mixture of isotopes, albeit a mixture that is 99% carbon with 6 neutrons in each nucleus.
An important series of isotopes is found with hydrogen atoms. Most hydrogen atoms have a nucleus with only a single proton. About 1 in 10,000 hydrogen nuclei, however, also has a neutron; this particular isotope is called deuterium. An extremely rare hydrogen isotope, tritium, has 1 proton and 2 neutrons in its nucleus. Figure (PageIndex{1}) compares the three isotopes of hydrogen.
The discovery of isotopes required a minor change in Dalton’s atomic theory. Dalton thought that all atoms of the same element were exactly the same.
Most elements exist as mixtures of isotopes. In fact, there are currently over 3,500 isotopes known for all the elements. When scientists discuss individual isotopes, they need an efficient way to specify the number of neutrons in any particular nucleus. The mass number (A) of an atom is the sum of the numbers of protons and neutrons in the nucleus. Given the mass number for a nucleus (and knowing the atomic number of that particular atom), you can determine the number of neutrons by subtracting the atomic number from the mass number.
A simple way of indicating the mass number of a particular isotope is to list it as a superscript on the left side of an element’s symbol. Atomic numbers are often listed as a subscript on the left side of an element’s symbol. Thus, we might see
[mathrm{^{mass: numberxrightarrow{hspace{45px}} 56}_{atomic: number xrightarrow{hspace{35px}} 26}Fe} label{Eq1}]
which indicates a particular isotope of iron. The 26 is the atomic number (which is the same for all iron atoms), while the 56 is the mass number of the isotope. To determine the number of neutrons in this isotope, we subtract 26 from 56: 56 − 26 = 30, so there are 30 neutrons in this atom.
Example (PageIndex{3})
How many protons and neutrons are in each atom?
- (mathrm{^{35}_{17}Cl})
- (mathrm{^{127}_{53}I})
How Many Protons In Iron
In (mathrm{^{35}_{17}Cl}) there are 17 protons, and 35 − 17 = 18 neutrons in each nucleus.
In (mathrm{^{127}_{53}I}) there are 53 protons, and 127 − 53 = 74 neutrons in each nucleus.
Exercise (PageIndex{3})
How many protons and neutrons are in each atom?
- (mathrm{^{197}_{79}Au})
- (mathrm{^{23}_{11}Na})
In (mathrm{^{197}_{79}Au}) there are 79 protons, and 197 − 79 = 118 neutrons in each nucleus.
In (mathrm{^{23}_{11}Na}) there are 11 protons, and 23 − 11 = 12 neutrons in each nucleus.
It is not absolutely necessary to indicate the atomic number as a subscript because each element has its own unique atomic number. Many isotopes are indicated with a superscript only, such as 13C or 235U. You may also see isotopes represented in print as, for example, carbon-13 or uranium-235.
Summary
The atom consists of discrete particles that govern its chemical and physical behavior. Each atom of an element contains the same number of protons, which is the atomic number (Z). Neutral atoms have the same number of electrons and protons. Atoms of an element that contain different numbers of neutrons are called isotopes. Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons.
Almost all of the mass of an atom is from the total protons and neutrons contained within a tiny (and therefore very dense) nucleus. The majority of the volume of an atom is the surrounding space in which the electrons reside. A representation of a carbon-12 atom is shown below in Figure (PageIndex{2}).
Concept Review Exercises
- Why is the atomic number so important to the identity of an atom?
- What is the relationship between the number of protons and the number of electrons in an atom?
- How do isotopes of an element differ from each other?
- What is the mass number of an element?
Answers
- The atomic number defines the identity of an element. It describes the number of protons in the nucleus.
- In an electrically neutral atom, the number of protons equals the number of electrons.
- Isotopes of an element have the same number of protons but have different numbers of neutrons in their nuclei.
- The mass number is the sum of the numbers of protons and neutrons in the nucleus of an atom.
Key Takeaways
- Each element is identified by its atomic number. The atomic number provides the element's location on the periodic table
- The isotopes of an element have different masses and are identified by their mass numbers.
Contributors and Attributions
Atomic Number 26 Periodic Table
Anonymous




